AG Prof. Rothermel: Neurophysiologie und Optogenetik

Forschungsschwerpunkte
We receive sensory information via our sensory organs. However, what we perceive (and what we don’t!) is strongly influenced by our experiences and expectations, as well as our attention and arousal state.
Our brain thereby actively influences and filters sensory information; mainly using so called “top-down” inputs. Failure to do so can lead to medical conditions, such as attention deficit disorder or autism. It is well established that modulations of information processing occur in higher brain areas such as neocortex and hippocampus. However, less is known about the mechanisms by which top-down inputs are affecting early processing stages of the brain. Modulations at these early stages are of particular importance since they will affect all subsequent processing steps.
Our lab investigates top-down modulations using the olfactory system (sense of smell) as a model. In the olfactory system top-down inputs to the earliest stage of information processing, the olfactory bulb, seem to be particular important as they even outnumber direct synaptic sensory inputs.
In order to investigate the mechanisms by which top-down inputs are affecting early sensory information processing and the specific behavioral contexts leading to their activation our lab uses a large variety of new optogenetic tools in combination with 2-photon microscopy and extracellular recordings.
To learn more about our research please don’t hesitate to contact us.
Methoden
In vivo calcium imaging
Neuronal networks (such as the olfactory bulb) consist of a lot of different cell types each of which having a specific function. In order to decipher the specific role of a single cell population we use calcium imaging and optogenetic approaches. Genetically encoded calcium indicators (GECIs) such as GCaMPs or RCaMPs enable us to detect calcium-induced fluorescent changes. Thereby, we can visualize neuronal activity in widefield and high resolution multi-photon microscopy (see below).
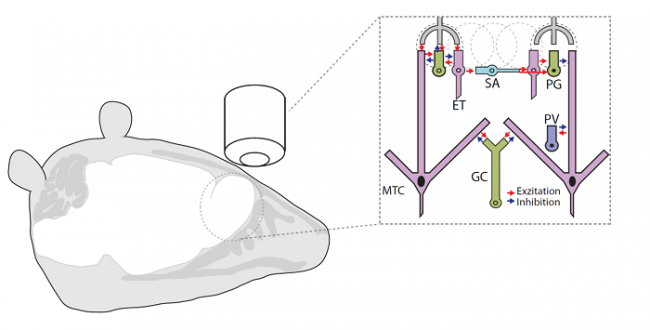
Calcium imaging in the olfactory bulb
Cell-type specific labelling
By using the Cre/LoxP-System we are able to label specific cell types with genetically encoded calcium indicators. Additionally, we can use this system to express optogenetic constructs (e.g. Channelrhodopsin or Halorhodopsin) in specific neuronal population. We currently have more than 10 different Cre-driver / reporter lines available in the lab. We either use transgenic (crossing cre-driver to cre-reporter lines) or viral-mediated tools (adeno-associated virus (AAV)) for markerprotein expression. Viral tools additionally allow us to trace neuronal populations anterogradely or retrogradely.
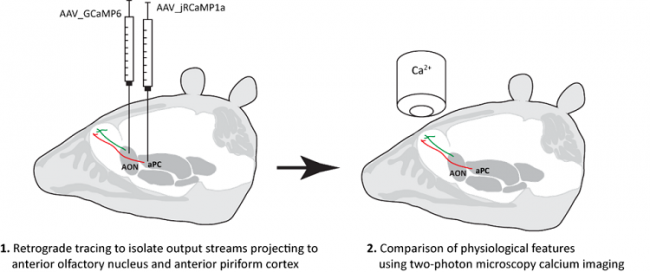
Retrograde tracing in the olfactory system
In vivo widefield microscopy
Widefield microscopy is a great tool to visualize neuronal activity at a large-scale population level. This technique allows us to visualize sensory evoked activity across the dorsal part of both olfactory bulbs simultaneously (Figure). The optical setup includes a Zeiss 5 x 0.17 NA objective and a scientific cMOS camera (PCO edge).
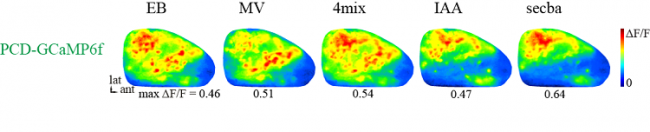
Topography of odorant representations (odor-maps)
In vivo high-resolution multi-photon microscopy
Widefield imaging is a great tool to measure neuronal activity on a large scale. In order to resolve activity of a large population of single cells we perform high resolution multi-photon microscopy. Our custom multi-photon setup (based on a Sutter MOM) is coupled to a pulsed Ti:Sapphire laser (Mai Tai HP, Spectra Physics) and controlled using Scanimage. Imaging is performed through a Nikon 16 x 0.80 W objective and the emitted light is collected by a gallium arsenide phospide/GaAsP photomultiplier tube (Hamamatsu). One great advantage of our microscopy systems is the direct and fast switch from widefield to multi-photon imaging. This gives us the opportunity to perform imaging at the population as well at the single cell level – in the same specimen under in vivo conditions.
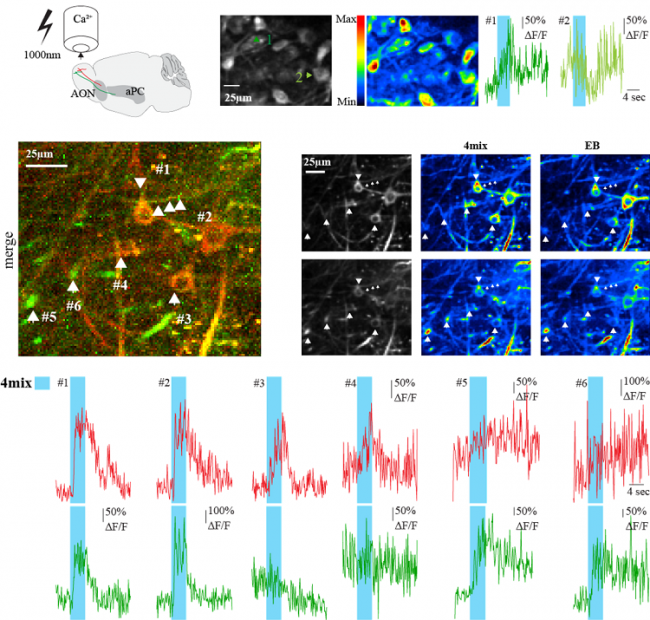
multi-photon laser scanning
Behavioral experiments
To investigate the behavioral relevance of top-down inputs we use different behavioral tasks. Our setup consists of a custom made olfactometer in combination with a “floating ball”. Thereby we can combine behavioral test with imaging and electrophysiological recordings.
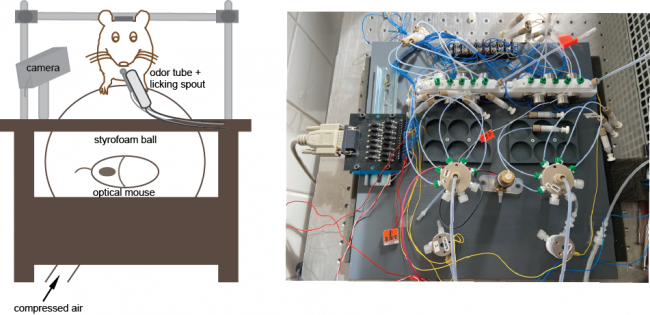
Behavior setup and olfactometer
Optogenetics
With the use of “optogenetics” it is possible to manipulate the activity of specific cell populations in order to decipher their specific function within a system or network. Viral tracing approaches allow us to perform a targeted opsin delivery. This way we are able to achieve a selective markerprotein expression in defined neuronal populations including their processes. We have a variety of opsins at our disposal, designed to either activate or inhibit the activity of neurons upon light-stimulation.
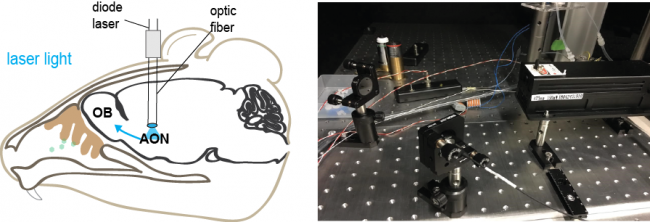
Optogenetic manipulations with DPSS Laser
In vivo electrophysiology
State-of-the-art multi-channel recordings allow us to measure spiking activity of multiple neurons simultaneously. These recordings allow us to investigate the modulation of sensory-evoked activity at a high temporal resolution. By recording activity using so called "multi tetrode arrays" (for more information visit the page of Erik Böhm) we are able to record the neuronal responses from different brain regions to a variety of sensory stimuli. So far, these arrays include up to 32 recording channels, allowing us to obtain a vast amount of data in the course of few recording sessions.
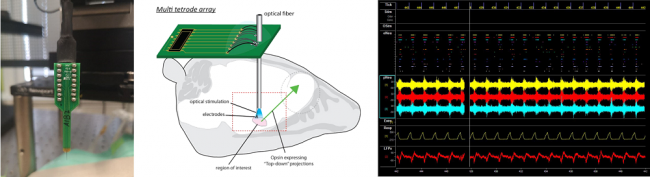
Multichannel recording system
Histology
Transgene expression is evaluated with post hoc histology in all experiments to confirm accurate targeting. Tissue sections are evaluated with a Leica TCS SP2 confocal laser scanning microscope at 10x or 20x magnification. The fluorescence intensity of confocal images is e.g. analyzed and plotted in ImageJ.
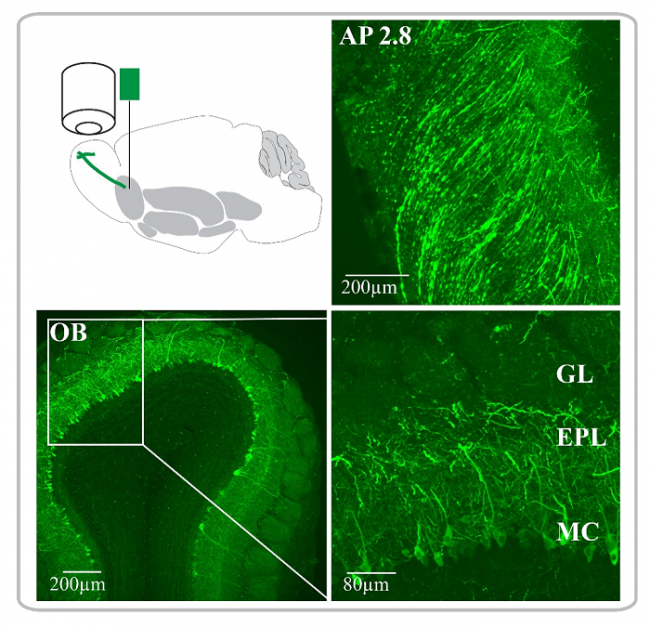
Histological reconstructions






